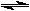 6 H2O + CuBr4-2
6 H2O + CuBr4-2The tube on the left contains only copper sulfate dissolved in solution. The tube on the right is the result of adding some potassium bromide solution. Given that the Cu(H2O)6+2 ion is blue and that the CuBr4-2 ion is green, answer the questions below.

a. What happened to the concentration of each of the ions when the KBr was added?
b. Explain why the solution changed color.
c. Would the tube feel hot or cold when the KBr was added?



