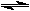 Mg+2 + 2 OH-
Mg+2 + 2 OH-The tube on the left contains Mg(OH)2(s) and water. A chemical has been added to cause the change shown in the tube on the right. Suggest a possibility for what chemical could have been added.

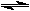 CH3OH + heat
CH3OH + heatIn an attempt to maximize the yield of methanol (amount of methanol produced), a chemist would try to shift the equilibrium as far to the right as possible. Which of the following would accomplish this?
-
a. heating the mixture
b. adding an excess of carbon monoxide
c. removing the methanol as it is formed
d. adding a substance that reacts with carbon monoxide



