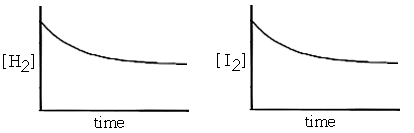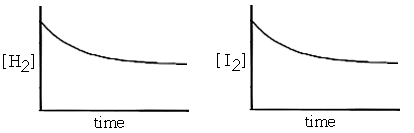Equilibrium and LeChatelier's Principle - Background
Suppose we put some hydrogen gas and iodine vapor in a sealed container. They will immediately begin to react with each other to form hydrogen iodide (the forward reaction). As they do, the concentration of hydrogen and iodine in the container will begin to drop (see graphs below - the brackets refer to concentrations). Since a decrease in concentration usually results in a decreased reaction rate, the forward reaction slows down.
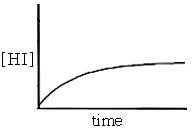
Initially there is no hydrogen iodide present so the reverse reaction does not occur. However, as the forward reaction proceeds the concentration of hydrogen iodide increases (see graph below). This increase in concentration causes the rate of the reverse reaction to increase as the reaction continues.
At some point the rate of the forward reaction will equal the rate of the reverse reaction. When this occurs the system is said to be in equilibrium.
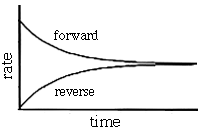
When a chemical system is in equilibrium a number of items are true:
The rates of the forward and reverse reactions are equal.
The concentrations of all the substances involved stop changing. This happens because they are being formed at the same rate that they are being consumed.
There will be both reactants and products present but the concentrations of them will not necessarily be equal. The fact that the forward and reverse reaction rates are the same does not mean the concentrations of the reactants and products are the same.
The system is a dynamic one. The reaction rates are equal, and the concentrations of the substances involved are not changing but there is still an interconversion of reactant and product.
While not immediately obvious, the same situation described above would occur if we had started with a sample of hydrogen iodide and no hydrogen or iodine. As the hydrogen iodide begins to decompose to form hydrogen and iodine, its concentration and the rate of the reverse reaction both drop. As hydrogen and iodine are formed, their concentrations and the rate of the forward reaction both increase. At some point the forward and reverse reaction rates will be equal and the system reaches equilibrium.
Continue with LeChatelier's Principle.