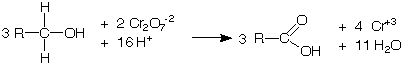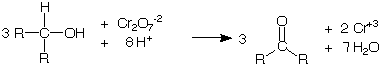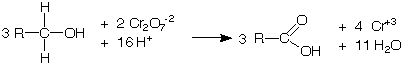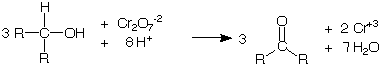Functional Groups - The Chromic Acid Test
Shows positive test for:
1o and 2o alcohols and aldehydes
Reactions:
aldehydes and primary alcohols are oxidized to carboxylic acids while the Cr+6 ion in the chromic acid is reduced to Cr+3.
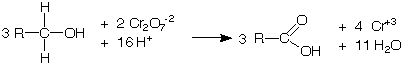
secondary alcohols are oxidized to ketones while the Cr+6 ion in the chromic acid is reduced to Cr+3.
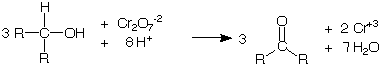
How to perform the test:
Three drops of the compound to be tested are mixed with 5 drops of acetone and 5 drops of chromic acid solution (an orange solution).
Indications of a positive test:
The disappearance of the red-orange color of chromic acid and the formation of a blue-green color of the Cr (III) ion indicates a positive test. Note that if the unknown is not soluble in water, two layers may be present. A blue-green color in either layer indicates a positive test.

a negative test (left) and a positive test (right)