(2) The stresses you can apply:
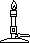 means heating the solution
means heating the solution means cooling the solution
means cooling the solution means adding a chemical (in this case KCl)
means adding a chemical (in this case KCl)When you apply the stress by clicking on the image, another picture will come up. On the left of the picture will be the original solution. On the right will be the solution after the stress has been applied.
(3) Any special comments you will need.